Control the danger of GPP flares like never before1-4
SPEVIGO® (SPESOLIMAB) IS INDICATED FOR THE TREATMENT OF FLARES IN ADULT PATIENTS WITH GENERALIZED PUSTULAR PSORIASIS (GPP) AS MONOTHERAPY
Treat GPP flares like never before1-4
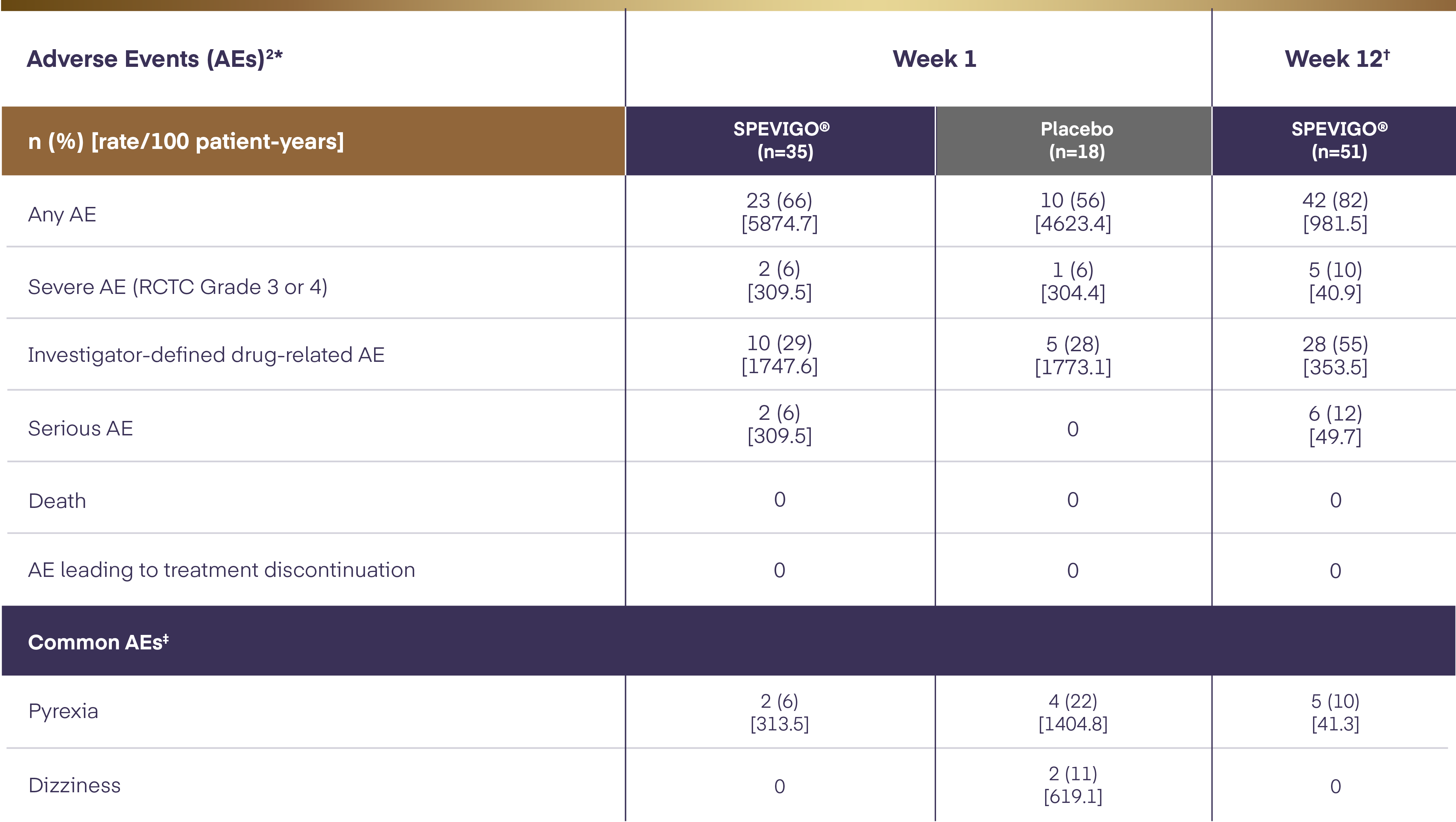
SPEVIGO® (spesolimab) is the first EMA-approved treatment for GPP flares.1,4 With SPEVIGO® (spesolimab), 54.3% of patients achieved complete pustular clearance* at Week 1.

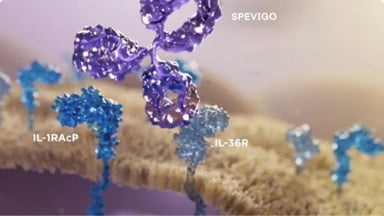
The first EMA-approved treatment to block the key inflammatory pathway in GPP by targeting the IL-36 receptor1,4,5
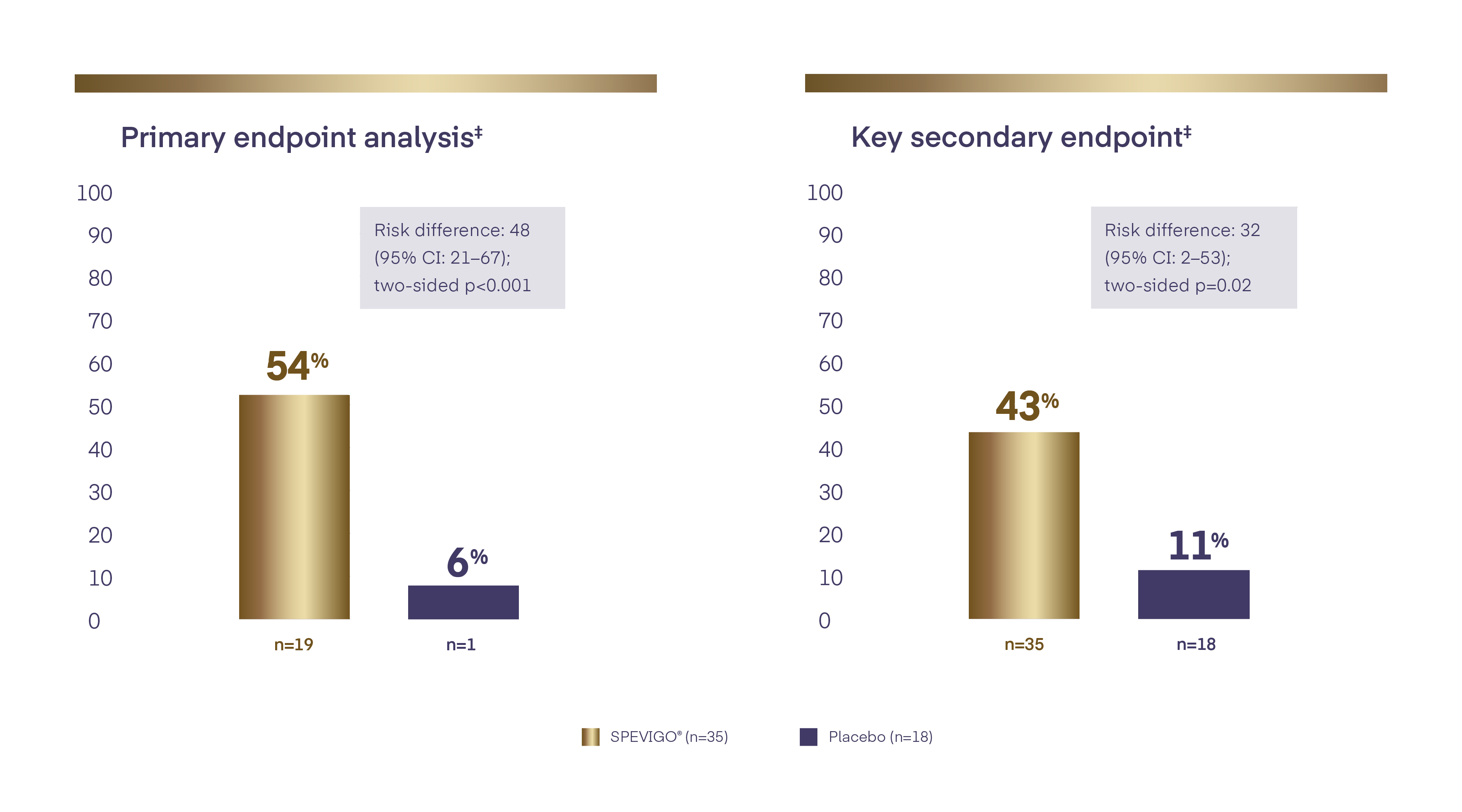
With SPEVIGO® (spesolimab), 54.3% of patients achieved complete pustular clearance* at Week1,4
* Pustular clearance defined as GPPGA pustulation subscore of 0 (no visible pustules).1
EMA=European Medicines Agency; GPPGA=Generalized Pustular Psoriasis Physician Global Assessment; IL-36=interleukin-36; MOA=mechanism of action.
References
-
SPEVIGO Summary of Product Characteristics. Boehringer Ingelheim Pharmaceuticals, Inc;2022.
-
Gooderham MJ, et al. Rev Clin Immunol. 2019;15(9):907-919.doi:10.1080/1744666X.2019.1648209
-
Ly K, et al. Psoriasis (Auckl).2019;9:37-42. doi:10.2147/PTT.S181808
-
Bachelez H et al. N Engl J Med 2021; 385:2431-2440. doi:10.1056/NEJMoa2111563
-
Navarini AA, et al. J Eur Acad Dermatol Venereol. 2017;31 (11):1792-1799.doi:10.1111/jdv.14386
GPP (07/2025) PC-GR-102302