SPEVIGO® (spesolimab)
Dosing and administration

The recommended dose of SPEVIGO® (spesolimab) is a single continuous IV infusion of 900 mg administered over 90 minutes1
If flare symptoms persist, an additional IV infusion may be administered 1 week after the initial dose.1
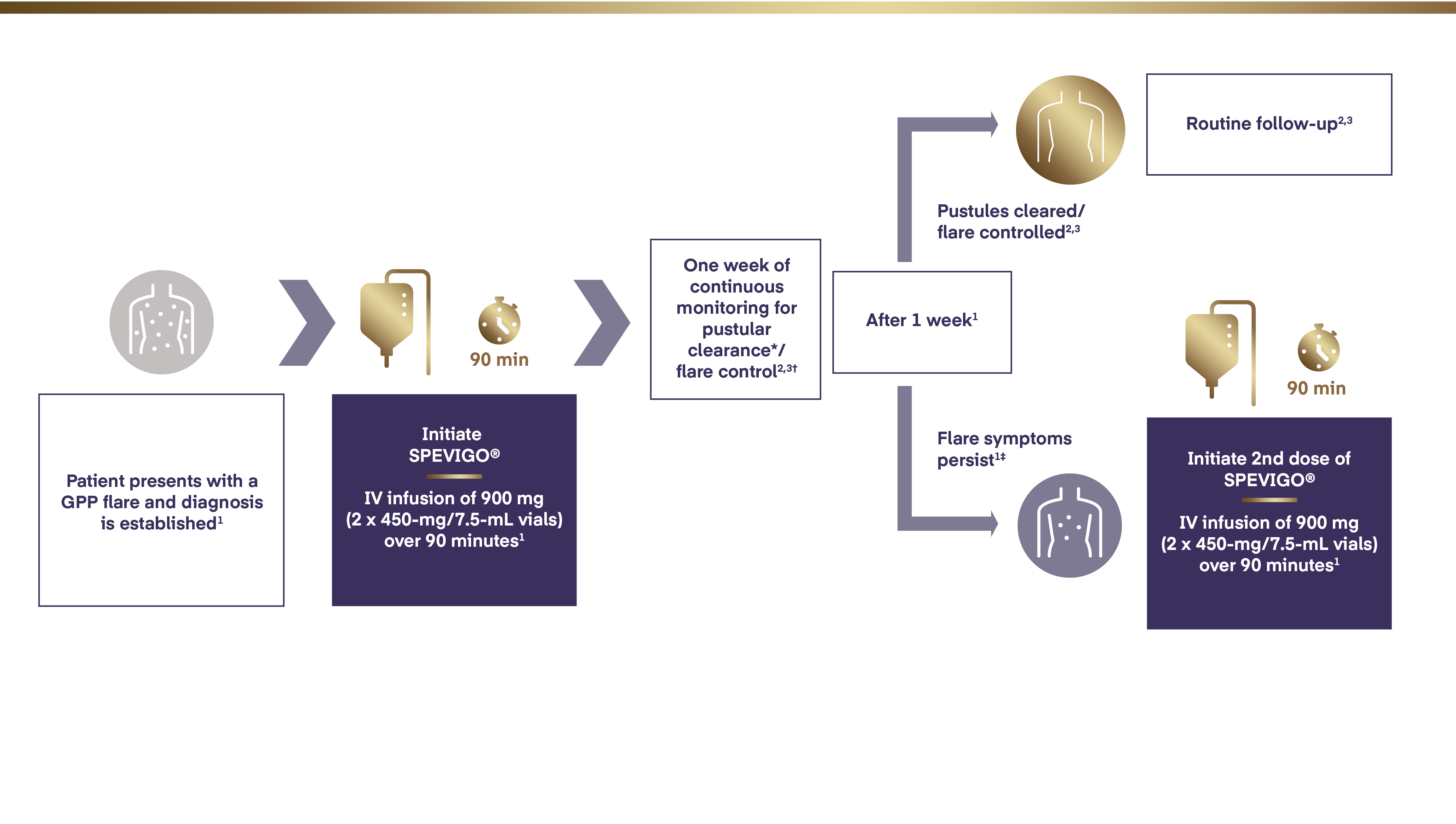
* Pustular clearance defined as GPPGA pustulation subscore of 0 (no visible pustules).1
† Flare control defined as GPPGA total score ≤1 and was reported for the 12-week study.1,3
‡ Flare persistence defined as GPPGA total score ≥2 and GPPGA pustulation subscore ≥2.3
GPP=Generalized Pustular Psoriasis; GPPGA=Generalized Pustular Psoriasis Physician Global Assessment; IV=intravenous.
The first EMA-approved treatment for Generalized Pustular Psoriasis (GPP) flares in adults1,3
|
References
-
SPEVIGO® Summary of Product Characteristics. Boehringer Ingelheim Pharmaceuticals, Inc.
-
Choon SE, Lebwohl MG, Marrakchi S, et al. BMJ Open. 2021;11(3):e043666. doi:10.1136/bmjopen-2020-043666
-
Bachelez H, Choon SE, Marrakchi S, et al; N Engl J Med. 2021;385(26):2431-2440. doi:10.1056/NEJMoa2111563
GPP (07/2025) PC-GR-102302



