SPEVIGO® (spesolimab)
SPEVIGO® (spesolimab) was evaluated for the treatment of GPP flares1

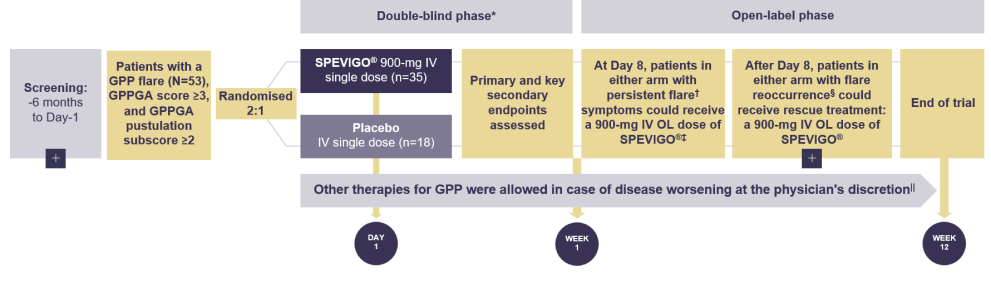
Effisayil™ 1 was a multicentre, randomised, double-blind, placebo-controlled trial of SPEVIGO® (Spesolimab) in patients with GPP
Effisayil™ 1 was a multicentre, randomised, double-blind, placebo-controlled trial of SPEVIGO® (spesolimab) in patients with GPP presenting with a flare1,2
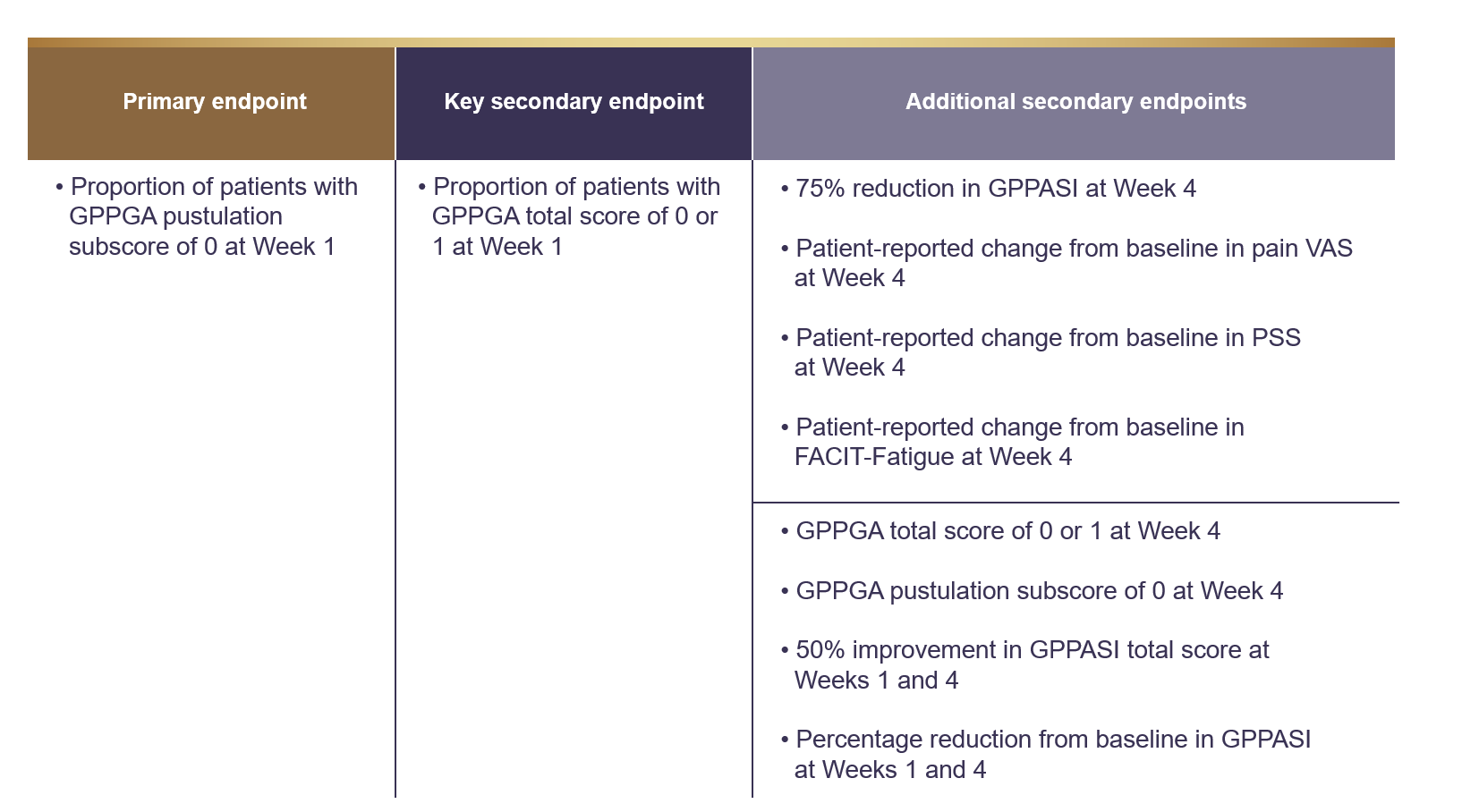
Primary endpoint1 : Proportion of patients with a GPPGA pustulation subscore of 0 (no visible pustules) at Week 1 after treatment
Key secondary endpoint1 : Proportion of patients with a GPPGA total score of 0 or 1 (clear or almost clear skin) at Week 1 after treatment
Selected exploratory endpoints1 : GPPGA pustulation subscore and GPPGA total score over time
-
*
Patients must have discontinued biologics, retinoids, methotrexate, and/or cyclosporine before receiving their first dose of SPEVIGO® (spesolimab) or placebo.2
-
†
Persistent flare defined as ≥2 GPPGA total score and ≥2 GPPGA pustulation subscore.2
-
‡
Patients who received other medications for GPP during Week 1 were not eligible for SPEVIGO® (spesolimab) at Day 8.3
-
§
Defined as a ≥2-point increase in GPPGA total score and GPPGA pustulation subscore ≥2 after achieving clinical response (GPPGA total score of 0 or 1).2
-
||
After Day 8, patients with a new flare (defined as a ≥2-point increase in GPPGA total score and pustulation subscore) could receive OL SPEVIGO® (spesolimab) if a GPPGA total score of 0 or 1 had been reached with SPEVIGO® (spesolimab) or placebo before.2
-
¶
At the end of Week 1, 2 patients on SPEVIGO® (spesolimab) and 1 patient on placebo had received ≥1 SoC therapy. From Day 8 to the end of the trial, 4 patients in each arm received ≥1 SoC therapy.2
GPP=Generalized Pustular Psoriasis; GPPGA=Generalized Pustular Psoriasis Physician Global Assessment; IV=intravenous; OL=open-label; SoC=standard of care.
Endpoint summary1,2
|
FACIT=Functional Assessment of Chronic Illness Therapy; GPPASI=Psoriasis Area and Severity Index for Generalized Pustular Psoriasis; PSS=Psoriasis Symptom Scale;
VAS=Visual Analogue Scale.
Patients aged 18 to 75 years with GPP, as defined by ERASPEN:
Primary, sterile, macroscopically visible pustules on non-acral skin (excluding cases in which pustulation is restricted to psoriatic plaques)
With or without systemic inflammation, with or without Plaque Psoriasis; can be either a relapsing condition (>1 episode) or a persistent condition (>3 months)
Evidence (or previous evidence) of systemic symptoms:
Fever
Asthenia
Myalgia
Elevated C-reactive protein level
Leukocytosis with peripheral blood neutrophilia (above ULN)
ERASPEN=European Rare and Severe Psoriasis Expert Network; ULN=upper limit of normal.
Patients were excluded if they presented with:
SAPHO syndrome
Plaque Psoriasis without pustules or with pustules restricted to psoriatic plaques
Drug-triggered AGEP
Immediate, life-threatening GPP flare or required intensive care treatment
Dose escalation of their maintenance treatment with cyclosporine, retinoids, or methotrexate within 2 weeks prior to randomisation
Treatment with any drug, including biologics and systemic drugs, considered likely to interfere with the safe conduct of the study or any prior exposure to an IL-36R inhibitor
AGEP=acute generalized exanthematous pustulosis; IL-36R=interleukin-36 receptor; SAPHO=synovitis-acne-pustulosis-hyperostosis-osteitis.
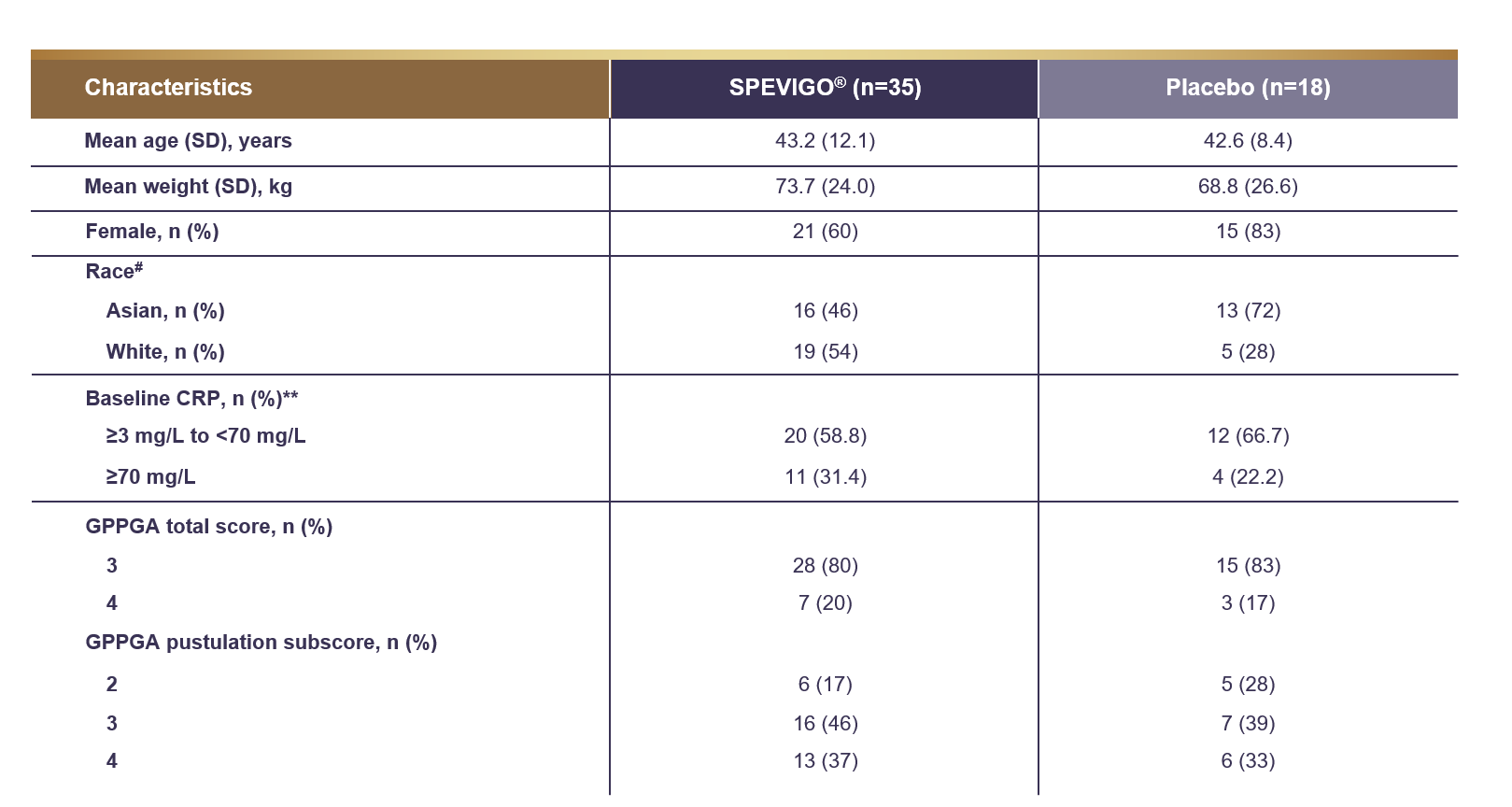
Baseline characteristics
In Effisayil™ 1, patient demographics and characteristics were evenly balanced between treatment arms2
|
†† Race was reported by the patient.1
‡‡ A total of 52 patients were included; 5 patients had missing values at baseline.1
CRP=C-reactive protein; SD=standard deviation.
The GPPGA score: Based on the PGA, modified by dermatology experts to measure specific GPP treatment outcomes3,5

# Flare control defined as GPPGA total score ≤1 and was reported for the 12-week study period.1
** To receive a score of 0 or 1, the patient should be afebrile, in addition to skin presentation requirements.6
PGA=Patient Global Assessment.
The GPPGA score: Based on the PGA, modified by dermatology experts to measure specific GPP treatment outcomes3,5
References
-
SPEVIGO® Summary of Product Characteristics. Boehringer Ingelheim Pharmaceuticals, Inc
-
Bachelez H et al. N Engl J Med. 2021;385(26):2431-2440. doi:10.1056/NEJMoa2111563
-
Choon SE et al. BMJ Open. 2021;11(3):e043666. doi:10.1136/bmjopen-2020-043666
-
Navarini AA et al. J Eur Acad Dermatol Venereol. 2017;31(11):1792-1799. doi:10.1111/jdv.14386
-
Burden AD et al. Am J Clin Dermatol. 2022;23(suppl 1):39-50. doi:10.1007/s40257-021-00653-0
GPP (07/2025) PC-GR-102302



